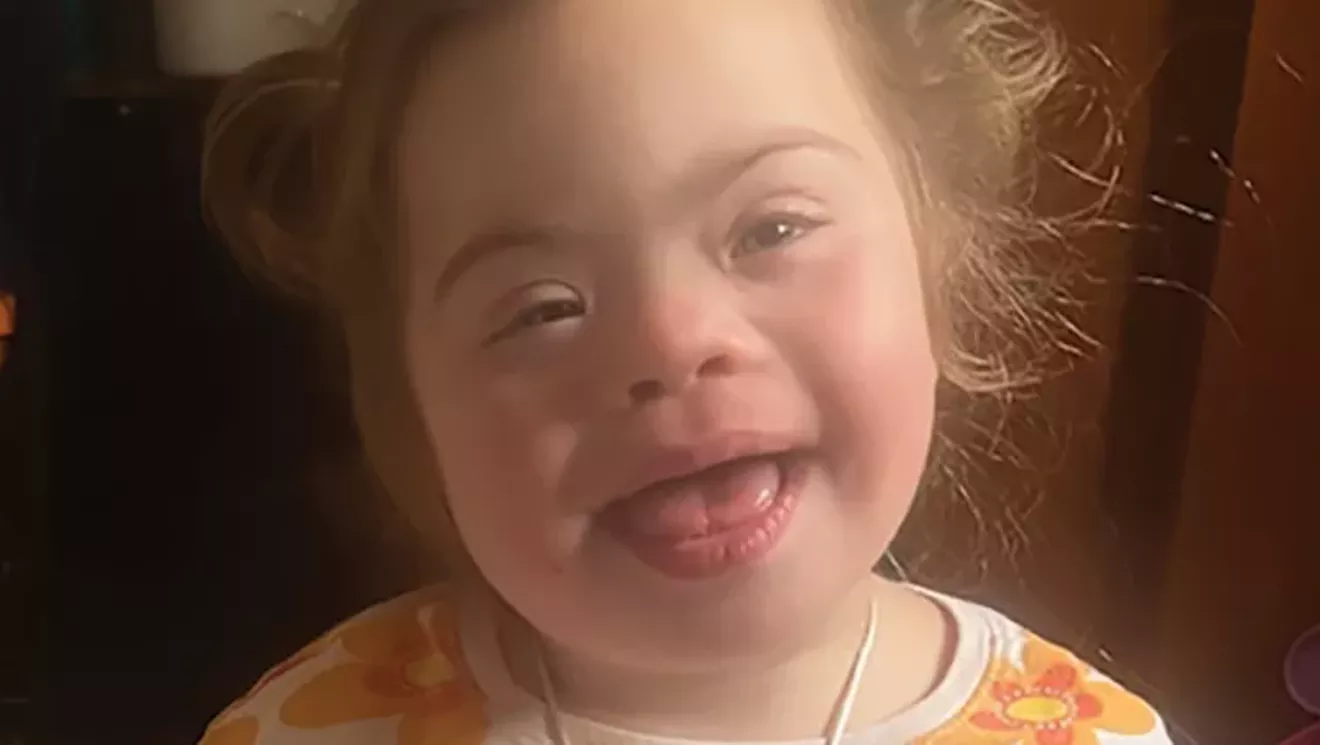
PHOTO COURTESY MILBURN FAMILY
Lottie Milburn is a 4-year-old in Springfield who has been diagnosed with a rare, life-threatening disease. Her parents hope to treat her with a promising gene therapy, but it hasn’t been approved by the FDA.
Lottie Milburn is a bright-eyed Springfield 4-year-old who brings joy wherever she goes.
“Lottie is hilarious. I feel like for somebody who really is not very verbal she communicates pretty well. She is really, truly, the sweetest little soul you’ve ever met,” her mother, Abby, told Illinois Times.
But in April, Abby and her husband, Steven, received the most devastating news any parent can hear. Their daughter has a rare, fatal disease. Only between 4,000 and 5,000 cases are known worldwide.
Lottie was diagnosed with Sanfilippo Syndrome, a genetic disease that affects the brain and spinal cord. It is caused by a problem with how the body breaks down certain large sugar molecules. In children with this condition, these sugar molecules build up in the body and eventually lead to damage of the central nervous system.
Symptoms of the disease rarely manifest themselves at birth. But as a child grows, they may begin having trouble learning and lose previously learned skills. As the disease progresses, children often develop seizures and movement disorders. Most children with Sanfilippo Syndrome die in adolescence or early adulthood.
In addition to Sanfilippo, Lottie was born with an unrelated condition: Down syndrome.
Abby was a teacher before becoming a stay-at-home mom for Lottie and her three siblings. Steven is a lawyer for the state of Illinois.
“We both grew up Catholic,” Abby said. “With everything going on, my husband has probably been better about reminding me that all of this just has to be in God’s plan – even if we don’t like it. It is hard.
“I think we trusted that from the beginning when we learned that Lottie would be born with Down syndrome. I know this sounds crazy, but I always had a feeling I would have a child with a disability, whether that was naturally or we adopted,” she said.
One of the hardest things the family has encountered is the federal bureaucracy that reviews whether medications should be approved for use within the U.S.
A promising drug to treat Sanfilippo recently finished its clinical trials and was submitted for approval to the U.S. Food and Drug Administration. The treatment developed by Ultragenyx is a gene therapy that is administered intravenously.
“It did very well in clinical trials,” Abby said. “There were 30 children (participating in the study). Everyone did well. They were able to prove… that either the regression slowed significantly or stopped. Children were regaining skills back that they had lost before the treatment. Some children who had stopped being able to feed themselves well were able to start independently feeding again. Cognitively, they noticed some speech and communication skills returning.”
Although it is experimental, this is the first treatment developed for Sanfilippo. But the FDA has yet to approve the medication, which leaves children like Lottie with few good options.
“We were just very hopeful that this would be something to help prolong Lottie’s life,” Abby said. “And not just prolong it, but do it in a meaningful way.”
This is a common predicament, said Brian Norman, director of state affairs for the Phoenix-based Goldwater Institute, a libertarian organization that lobbies for streamlining the approval of medications.
The FDA has prided itself in protecting the public from medications that could have had harmful consequences. For example, in the 1950s and 1960s European regulators approved the sedative thalidomide, while the FDA did not. Ultimately, thousands of European children whose mothers took the medication during their pregnancies were born with missing limbs but American children were spared this tragedy.
But Norman said the FDA’s cautious approach has a downside.
“They never bring up the invisible graveyard of individuals who are not able to access promising investigational treatments because of their slow-moving nature,” he said. “Being overly cautious comes at a cost. … Some individuals are going to lose, and oftentimes it’s terminally ill patients who end up paying the ultimate cost.”
To work around the bureaucratic inertia, 41 states and Congress passed the original “Right to Try” law, which allows patients with terminal illnesses, in some instances, to access investigational treatments that have not yet received the FDA’s approval.
Norman said the foremost authority on these laws is Naomi Lopez, who ironically lives in Springfield and only about a block from the Milburns’ home.
In 2017, Lopez testified before Congress in favor of the “Right to Try” legislation that was signed into federal law in 2018 by President Donald Trump.
Lopez has lobbied 30 state legislatures and has been working with the Goldwater Institute on the newer Right to Try for Individualized Treatments laws, which offer a new pathway for rare patients to access bespoke treatments. That law has now passed in 16 states and will eventually be introduced in Congress.
Lopez and the Milburns do not know each other but said they are eager to meet.
Under the federal Right to Try law, “The child’s physician would have to be recommending the treatment, and the manufacturer would have to agree to be providing the treatment,” Lopez said. “If everyone is onboard and the treatment has passed the basic safety trial, they actually don’t have to get the FDA’s permission to proceed with treatment.”
Sometimes gene therapies call for making a custom medication for just one patient, Lopez said. This can be done more easily in states that have the Right to Try for Individualized Treatments laws on the books. Illinois is not one of them. But neighboring Iowa and Indiana are, she said.
The Milburns are investigating options and are considering reaching out to members of Congress. They have expressed frustration with the FDA.
“Sometimes it can take upwards of a decade to get a drug from start to finish through approval,” Norman said. “The FDA is risk averse. It doesn’t really take into account the urgency faced by individual patients. … The FDA, generally speaking, is a slow-moving behemoth that is not responsive to the needs of individual patients who are suffering with their terminal illnesses or life-threatening illnesses on a day-to-day basis.”